Inhibition of human cancer cell line
growth and human umbilical vein endothelial cell angiogenesis by artemisinin
derivatives in vitro
Pharmacological Research 48 (2003)
231–236
Huan-Huan Chen*, Hui-Jun Zhou, Xin Fang
Department of
Pharmacology and Toxicology, College of Pharmacology, Zhejiang University,
Hangzhou, Zhejiang 310031, PR China
Accepted 30 March 2003
Abstract
Artemisinin derivatives artesunate (ART) and
dihydroartemisinin are remarkable anti-malarial drugs with low toxicity to
humans. In the present investigation, we find they also inhibited tumor cell
growth and suppressed angiogenesis in vitro. The anti-cancer activity was
demonstrated by inhibition (IC 50) of four human cancer cell lines:
cervical cancer Hela, uterus chorion cancer JAR, embryo transversal cancer RD
and ovarian cancer HO-8910 cell lines growth by the MTT assay. IC50
values ranged from 15.4 to 49.7 µM or from 8.5 to 32.9 µM
after treatment with ART or dihydroartemisinin for 48 h, indicating that
dihydroartemisininwasmore effective than ART in inhibiting cancer cell lines.
The anti-angiogenic activities were tested on in vitro models of angiogenesis,
namely, proliferation, migration and tube formation of human umbilical vein
endothelial (HUVE) cells.We investigated the inhibitory effects of ART and
dihydroartemisinin on HUVE cells proliferation by cell counting, migration into
the scratchwounded area in HUVE cell monolayers and microvessel tube-like
formation on collagen gel. The results showed ART and dihydroartemisinin
significantly inhibited angiogenisis in a dose-dependent form in range of
12.5–50 µM and 2.5–50 µM, respectively. They indicated
that dihydroartemisinin was more effective than ART in inhibiting angiogenesis
either. These results and the known low toxicity are clues that ART and
dihydroartemisinin may be promising novel candidates for cancer chemotherapy.
© 2003 Elsevier Science Ltd. All rights reserved.
1. Introduction
Artemisinin and its derivatives such as artesunate
(ART) and dihydroartemisinin distinguish themselves as a new generation of
anti-malarial drugs with low toxicity. Having been used for the treatment of
more than one million cases of malarial infection, artemisinin and its analogs
are considered as safe drugs with no obvious adverse reaction or noticeable
side effects [1]. Especially, artemisinin
derivatives exert remarkable activity against otherwise drug-resistant
plasmodium falciparum and plasmodium vivax strains [2,
3, 4]. Thus, they are gaining
increasing importance in the treatment of malarial infection.
Recently, it is reported that the anti-malarial
artemisinin derivatives are also active against tumor cells. Some investigators
found artemisinin drugs had inhibitory effects on cancer cell growth such as
lung, malanomas, breast, renal, prostate, CNS cancer cells including many
drug-resistant cancer cells [5, 6]. Moreover, they also have suppressive effects on the
growth of human tumor xenografts in rat, mice and nude mice [7, 8]. These studies indicated that
artemisinin derivatives have anti-cancer activities in vitro and in vivo.
It is known that tumors are angiogenesis dependent and
can elicit the production by a new capillary endothelium from the host by
themselves [9]. Angiogenesis, the proliferation and
migration of endothelial cells (ECs) resulting in the formation of new blood
vessels, is a vital process for the progression of all solid tumors from a
small, localized focus to an enlarging tumor with the capability to metastasize
[10, 11]. Consequently,
inhibition of angiogenesis may lead to control of tumor growth and metastasis
[12]. In cancer therapy, cancer inhibitors which
have the anti-angiogenic activity as well as anti-cancer activity may kill
cancer much more effectively. Artemisinin derivatives have been suggested to
have anti-tumor activity. However, anti-angiogenic activity has not yet been
demonstrated.
In the present study, we have investigated whether
artemisinin derivatives ART and dihydroartemisinin have the anti-angiogenic
activity as well as the anti-tumor activity.We tested the inhibitory effects of
ART and dihydroartemisinin on human cervical cancer Hela, uterus chorion cancer
JAR, embryo transversal cancer RD and ovarian cancer HO-8910 cell lines growth
and the proliferation, migration, tube formation of human umbilical vein
endothelial cells (HUVECs).
2. Materials and methods
2.1. Materials
ART was purchased from Guiling Pharmaceutical Co.
(Guangxi, China) and dihydroartemisinin was a gift from the Engineer, Liuxu of
Guiling Pharmaceutical Co. Collagen (Type I) was purchased from Sigma (Bornem,
Belgium), RPMI 1640 medium, Dulbecco’s modified eagle’s medium (DMEM)
were supplied by Gibco (BRL, Merelbeke, Belgium). HEPES, DMSO, penicillin,
streptomycin, MTT and EC growth factor (VEGF) were obtained from Sigma Chemical
Co. (St. Louis, MO, USA).
Four human cancer cell lines: cervical cancer Hela,
uterus chorion cancer JAR, embryo transversal cancer RD, ovarian cancer HO-8910
cell lines and fibroblast cell line NIH-3T3 were purchased from Shanghai
Institutes for Biological Sciences (Shanghai, China). HUVECs were obtained from
the American Type Culture Collection (ATCC, Rockville, MD).
2.2. Cell culture
The four human cancer cell lines: Hela, JAR, RD, HO-
8910 and NIH-3T3 cells were cultured in RPMI 1640 medium supplemented with 10%
fetal calf serum and antibiotics (100 IU ml -1 penicillin and 100
µg ml-1 streptomycin), at 37 °C, 5% CO2 in air.
HUVECs were grown in DMEM medium with 10% FCS, 10 ng ml-1VEGF and
antibiotics. HUVECs were used within 10 passages. Human endometrium cells were
isolated from the uterus of misbirth woman by 0.05% trypsin digestion and
cultured in DMEM medium with 15% FCS. Cells were used within seven passages.
Human endometrium cells were cultured and checked by the technologist of
Shanghai Institutes for Biological Sciences.
2.3. Growth assay of various types of cells
Four cancer cells, NIH-3T3 cells and endometrium cells
were plated in 24-well plates at a density of 1×104 cells per
well. After 24 h of culture in the normal growth medium, cells were exposed to
graded concentrations of ART or dihydroartemisinin for 48 h. Cells were then
incubated with 5 mg ml-1MTT solution for 4 h. One hundred microliter
of 10% sodium dodecyl sulfate solution was added to the culture. Absorbance at
570 nm was determined by using an ELISA reader (Bio-Tek instruments, Inc.,
USA). By the MTT method, cell numbers were obtained as absorbance values. The
results were expressed as IC50values (50% inhibitory concentration).
2.4. Growth inhibition assay of HUVECs
HUVECs were seeded at a density of 1
×104 cells per well into 24-well plates. After 24 h incubation
at 37 °C in a 5% CO2 incubator, ART or dihydroartemisinin at
different concentrations were added to the wells and the cells were further
cultured for 48 h. The number of cells was counted with a coulter counter. Cell
viability was evaluated by the trypan blue exclusion test.
2.5. HUVEC migration assay
5 ×104 HUVECs per well were seeded
into 24-well plates and grown to confluence. The ‘scratch wound’ in
the confluence monolayers was made using a razor blade, then each well was
rinsed with PBS and 10% FBS–DMEM medium containing ART or
dihydroartemisinin were added. The plates were incubated at 37 °C, 5%
CO2 in air for 24 h. The number of cells that had migrated from the
edge of the wound in each 250 µm × 500 µm area of 10 randomly
chosen fields was counted. Results were expressed as the average number of
cells per field.
2.6. Cell microvessel formation assay
HUVEC differentiation was evaluated by using a tube
formation method as described previously [13,
14] with minor modifications. An 8:1:1 volume of 3mg
ml–1 Type I collagen, DMEM (10×), 0.1M NaOH + 0.2 M HEPES
+ 0.26 M Na2 CO3 was made and poured into 24-well plates
with 750 µl per well at 4 ° C. After a collagen gel formed by
incubating at 37 °C for 1 h, 1 × 104 HUVECs per well were
seeded on the collagen-coated wells and incubated for 24 h. Subsequently, 10%
FBS–DMEM supplemented with 10 ng ml–1 VEGF containing ART
or dihydroartemisinin were added into the wells. After incubation for 3 days,
microvessel formation was observed using a light microscopy and photographed.
The total lengths of the tubular structures in three randomly chosen
microscopic fields per well were measured by making use of a curvimeter in the
microscope (Olympus, Tokyo, Japan).
2.7. Data analysis
All values are expressed as the mean ±S.D. and
the significant levels between two groups were assessed by Student’s
t-test. P values less than 0.05 were considered to be
statistically significant.
3. Results
3.1. Effect of ART and dihydroartemisinin on the
growth of various types of cells
Both ART and dihydroartemisinin inhibited the growth
of the four cancer cell lines in a concentration-dependent 233 manner by the
MTT assay. Treatment with ART and dihydroartemisinin at concentrations greater
than 5 µM for 2 days reduced the cell growth of all four lines at
different levels. RD cell line was the most sensitive to ART and
dihydroartemisinin in this test panel, and can be inhibited 89.7% or 94.5% by
120 µM ART or 120 µM dihydroartemisinin, respectively. The other
cancer cell lines can be inhibited above 80% by 120 µM ART or 120
µM dihydroartemisinin (data not shown). The IC50of the four
cancer cell lines by ART and dihydroartemisinin was shown at Table 1.
Table 1
|
Inhibition (IC50 ) of four cancer
cell lines by ART and dihydroartemisinin |
|
Substance |
Cell line, IC50 (µ M)
|
|
|
Hela |
JAR |
RD |
HO-8910 |
|
ART |
38.6 ±4.3
|
40.4
±5.8 |
15.4
±1.0 |
49.7 ±6.9
|
|
Dihydroartemisinin |
15.7 ± 3.7
|
24.5 ±
5.3 |
8.5 ±
1.1 |
32.9 ±
4.7 |
|
|
|
|
|
|
|
|
|
|
|
Various cells were cultured
with ART or dihydroartemisinin for 48 h and cell growth was assessed by the MTT
colorimetric method. Results of experiments in triplicate are expressed as the
IC50 values that suggest the inhibitory activity of ART and
dihydroartemisinin against four cancer cell lines. |
We showed that dihydroartemisinin was more effective
in inhibiting all the four cell lines growth than ART.
We also tested the effects of ART and
dihydroartemisinin on the proliferation of the normal control cells. The
IC50 values for NIH-3T3 cells and human endometrium cells were
105.77 µM or 69.56 µM for ART or dihydroartemisinin and 139.4
µM or 88.02 µM for ART or dihydroartemisinin, respectively.
3.2. Effects of ART and dihydroartemisinin on
proliferation of HUVECs
Effects of ART and dihydroartemisinin on proliferation
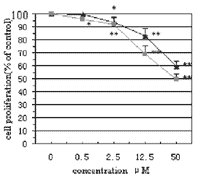
of HUVECs were observed (Fig. 1). Cell proliferation was inhibited in a
concentration-dependent manner. At every concentration >0.5 µM, the
groups of treatment with ART and dihydroartemisinin were significantly
different when compared with each other (P < 0.05).
Dihydroartemisinin was more effective than ART in inhibiting the cell growth.
At the highest concentration of 50 µM ART and dihydroartemisinin had
inhibition rates of about 40 and 50%, respectively.
The above results show that the IC50values
for HUVEC and four human cancer cell lines were all lower than those for
fibroblast cells and human endometrium cells, indicating that the growth
inhibition activity of ART and dihydroartemisinin against HUVEC and the four
cancer cell lines was stronger than fibroblast cells and human endometrium
cells.
|
|
|
|
|
|
Fig. 1.
Quantification of inhibitory effects of ART and dihydroartemisinin on HUVECs.
HUVECs were plated in 24-well plates, allowed to attach for 24 h and then
treated with different concentrations of ART or dihydroartemisinin for 2 days.
Cell proliferation was determined by cell counting. Data represent the average
(± S.D.) of three experiments. Symbols indicate ART
 and
dihydroartemisinin and
dihydroartemisinin
 . (*) P
< 0. 05; (**) P < 0. 01, compared to
control. . (*) P
< 0. 05; (**) P < 0. 01, compared to
control. |
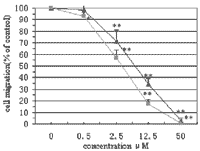
Fig. 2.
Quantitative
inhibition of HUVECs by ART and dihydroartemisinin. Confluent cultures of
HUVECs were wounded with a razor blade. The cells were incubated with ART or
dihydroartemisinin at different concentrations for 24 h. The numbers of cells
migrated from the edge of the wound within each 125 µm × 500
µm area were counted. Data represent the average (± S.D.) (n
= 3). Symbols indicate ART
 and
dihydroartemisinin and
dihydroartemisinin
 . (*) P
< 0.05; (**) P < 0.01, compared to control. . (*) P
< 0.05; (**) P < 0.01, compared to control. |
3.3. Effects of ART and dihydroartemisinin on
migration of HUVECs
On HUVECs, either ART or dihydroartemisinin induced a
dose-dependent decrease in cell migration (Figs. 2 and 3). Compared to the
inhibition of cell growth, the effect was evident from lower concentrations.
ART and dihydroartemisinin suppressed cell migration slightly at a
concentration of 0.5µM and inhibited it completely at 50 µM.
Dihydroartemisinin was more effective than ART (P < 0.01).
3.4. Effects of ART and dihydroartemisinin on
HUVEC tube formation
We tested the effects of ART and dihydroartemisinin on
HUVEC tube formation in vitro. Tubulogenesis was induced in vascular ECs by
seeding them on the surface of the collagen (Type I) gel for 24 h. Fig. 4 shows
the branching vessel-like structures formed by HUVECs. When ART or
dihydroartemisinin was added to the culture, there was a decrease in both the
number and length of tube formation in a dose-dependent manner (Fig. 5). There
was approximately 70 or 90% reduction in the total tube length per field
following 50 µM ART or dihydroartemisinin treatment for 48 h,
respectively. The inhibitory activity of dihydroartemisinin is also greater
than that of ART (P < 0.01).
|
a. 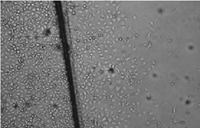 |
a. 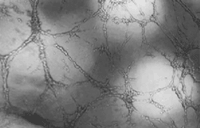 |
|
b. 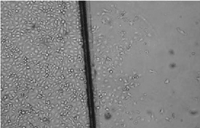 |
b. 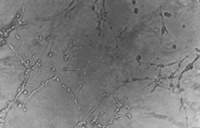 |
|
c.  |
c. 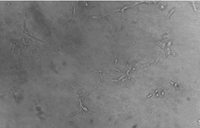 |
|
Fig. 3.
Effect of
dihydroartemisinin on HUVECs migration. Microscopic morphology (200×) of
HUVECs treated as in Fig. 2 : (a) control; (b) 2.5 µ M
dihydroartemisinin; (c) 50µ M dihydroartemisinin. |
Fig. 4.
Effect of
dihydroartemisinin on HUVEC tube formation. Microscopic morphology (200×)
of HUVECs treated as in Fig. 5 : (a) control; (b) 12.5 µ M
dihydroartemisinin; (c) 50 |
|
|
|
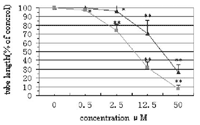
Fig. 5.
Dose-dependent inhibition of HUVEC tube formation by ART and
dihydroartemisinin. HUVECs were plated in a three-dimensional culture system on
collagen gels and then treated by ART or dihydroartemisinin at different
concentrations for 2 days. Total length of tube formation per field was
measured and results were expressed as percent of control (average ±S.
D. ) (n = 3). Symbols indicate ART
 and
dihydroartemisinin and
dihydroartemisinin
 . (*) P
< 0. 05; (**) P < 0. 01, compared to control. . (*) P
< 0. 05; (**) P < 0. 01, compared to control.
|
4. Discussion
Although some studies have shown that the
anti-malarial ART and dihydroartemisinin were active against many cancer cell
lines in vitro [5, 6, 15], their effects on these four cancer cell lines Hela,
JAR, RD and HO-8910 were not reported. In this investigation, we examined ART
and dihydroartemisinin’s anti-tumor activity on the above four cancer cell
lines to extend the anti-tumor spectrum of the two drugs. The
IC50values of these four cell lines were different according to
their different sensitivities towards ART and dihydroartemisinin. Ovarian
cancer line showed the highest IC50values indicating the lowest
sensitivity to both ART and dihydroartemisinin in this test panel. While,
either ART or dihydroartemisinin was most active against embryo transversal
cancer cell line RD. Compared to ART, dihydroartemisinin had greater anti-tumor
activity in vitro.
Angiogenesis plays a vital role in tumor growth,
intrasavation, metastatic spread [10, 11]. Inhibition of angiogenesis provides a good chance of
preventing cancer from becoming malignant [16, 17]. Angiogenesis is composed of several process
dissociations of pericytes from preexisting vessel, digestion of extracellular
matrix with proteases growth, migration and invasion of ECs, tube formation,
then finally remodeling occurs. Among these processes, growth, migration and
tube formation of ECs are essential for angiogenesis. This motivates us to
determine anti-angiogenic activities of ART and dihydroartemisinin by
inhibiting HUVECs growth, migration and tube formation. Our data showed that
the inhibition of HUVECs growth of dihydroartemisinin occurred at higher
concentration than the concentrations needed to inhibit cell migration and tube
formation. It was expected that the suppression of angiogenesis by
dihydroartemisinin might not be induced only by inhibiting ECs proliferation.
The mechanism of such effect should be studied further. In the present
investigation of anti-angiogenic activity, results suggested that
dihydroartemisinin and ART were two potent inhibitors. It was known that
dihydroartemisinin was the main product of artemisinin and its derivatives
including ART by metabolization of human bodies.
This information together with our results indicated
ART and other artemisinin drugs might continue to be active or even more
against cancer after metabolization in human bodies. The mechanism of
inhibition of ART and dihydroartemisinin on tumor growth is not studied
exhaustively. It is well known that the artemisinin and its derivative
molecules contain an endoperoxide bridge that reacts with a ferrous iron atom
to form free radicals which contributes to their anti-malarial activity [18, 19]. However, whether the formation
of radical molecules and/or reactive oxygen species of artemisinin drugs
contributes to their anti-tumor activity is not completely proved. Moreover, it
is not known whether genetic pathways are involved in cancer cells and to which
extent they vary in different derivatives [20,
21]. Singh and Lai have shown that dihydroartemisinin
and ART are selectively toxic to human cancer cells and with relatively low
toxicity on normal human cells [22, 23]. It is also reported that artemisinin derivatives are
active against many drug-resistant cancer cell lines, such as small-cell lung
cancer (SCLC) [24]. Compared to normal cells,
cancer cells contain higher rates of iron intake correlated with their high
transferrin receptor concentration. So, cancer cells including drug-resistant
cancer cells are more susceptible to artemisinin drugs under conditions of high
iron availability [25, 26].
Although we suggested the inhibitory effects of ART
and dihydroartemisinin on angiogenesis in vitro, the mechanism of inhibition is
still not clear at the present time and further studies are needed to gain a
full understanding of the anti-angiogenic activity in vivo. Since many
identified tumor and angiogenesis inhibitors have problems concerning their
therapeutic applications because of their excessive toxicity and limited
efficacy. The anti-tumor and anti-angiogenic efficacy together with the known
low and selective toxicity make it possible that ART and dihydroartemisinin may
be promising novel candidates for cancer chemotherapy.
Acknowledgements
This work was supported in part by a Grant-in-Aid for
new drug research from National Key Laboratory of Chinese Academy of Sciences
and by funds for Scientific Research from Zhejiang University.
References
[1] Klayman DL. Qinghaosu
(artemisinin): an antimalarial drug from China. Science 1985;228:1049–55.
[2] Benakis A, Paris M, Loutan L,
Plessas CT, Plessas ST. Pharmacokinetics of artemisinin and artesunate after
oral administration in healthy volunteers. Am J Trop Med Hyg
1997;56:17–23.
[3] Hien TT, White NJ. Qinghaosu.
Lancet 1993;341:603–8.
[4] Dhingra V, Rao VM, Narasu L.
Current status of artemisinin and its derivatives as anti-malarial drugs. Life
Sci 2000;66:279–300.
[5] Efferth T, Dunstan H, Sauerbrey A,
Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against
cancer. Int J Oncol 2001;18:767–73.
[6] Efferth T, Davey M, Olbrich A,
Rücher G, Gebbart E, Daveu R. Activity of drugs from traditional Chinese
medicine towards sensitive and MDRI- or MRPI-overexpressing multidrug-resistant
human CCRF-CEM leukenna cells. Blood Cells Mol Dis 2002;28:160–8.
[7] Yang X, Pan Q, Liang Y-G. Cancer
1997;16:186–7 (in Chinese).
[8] Wang Q, Wu L-M, Li A-Y, Zhao Y,
Wang N. China J Chinese Materia Med 2001;26:707–8 (in Chinese).
[9] Folkman J. Tumor angiogenesis:
therapeutic implications. N Engl J Med 1971;285:1182–6.
[10] Folkman J, Klagsburn M.
Angiogenic factors. Science 1987;235: 442–7.
[11] D’Amore PA, Thompson RN.
Mechanism of angiogenesis. Annu Rev Physiol 1987;49:453–64.
[12] Kim KJ, Li B,Winer J, Armanini
M, Gillett N, Phillips HS, Ferrata N. Inhibition of vascular endothelial growth
factor-induced angiogenesis suppresses tumor growth in vivo. Nature
1993;362:841–4.
[13] Kawasaki J, Hirano K, Hirano M,
Nishimura J, Nakatsuka A, Fujishima M, Kanaide H. Dissociation between the Ca 2
+ signal and tube formation induced by vascular endothelial growth factor in
bovine aortic endothelial cells. Eur J Pharm 2000;398:19–29.
[14] Soeda S, Kozako T, Iwata K,
Shimèno H, Malingre TM, Feraly FS, Kampinga HH, Kenings AW. Oversulfated
fucoidan inhibits the basic fibroblast growth factor-induced tube formation by
human umbilical vein endothelial cells: its possible mechanism of action.
Biochim Biophy Acta 2000;1497:127–34.
[15] Woerdenbag HJ, Moskal TA, Pras
N, Kishimoto S, Sudo K, Kanamaru T, Brem H. Cytotoxicity of artemisinin-related
endoperoxides to Enrlich ascites tumor cells. J Nat Prod 1993;56:849–59.
[16] Ingber D, Fujita T, Folkman J,
et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress
tumor growth. Nature 1990;348:555–7. 236
[17] O’Reilly MS, Holmgren L,
Shing Y, Chen CC, Rosenthal RA, Moser M, Lane WS, Cao Y, Sage EH, Folkman J.
Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of
metastases by a Lewis lung carcinoma. Cell 1994;79:315–28.
[18] Berman PA, Adams PA. Artemisinin
enhances heme-catalysed oxidation of lipid membranes. Free Radic Biol Med
1997;22:1283–8.
[19] Zhang F, Grosser DK, Meshnick
SR. Hemin-catalyzed decomposition of artemisinin (qinghaosu). Biochem Pharmacol
1992;43:1805–9.
[20] Efferth T, Olbrich A, Bauer R.
mRNA expression profiles for the response of human tumor lines to the
anti-malarial drugs artesunate, arteether, and artemether. Biochem Pharmacol
2002;64:617–23.
[21] Kihara C, Tsunoda T, Tanaka T,
Yamana H, Furukawa Y, Ono K, Kitahara O, Zembutsu H, Yanagawa R, Hirata K,
Takagi T, Nakamura Y. Prediction of sensitivity of esophageal tumor to adjuvant
chemotherapy by cDNA microarray analysis of gene-expression pro- files. Cancer
Res 2001;61:6474–9.
[22] Singh NP, Lai H. Selective
toxicity of dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci 2001;79:49–56.
[23] Lai H, Singh NP. Selective
cancer cell cytotoxicity from exposure to dihydroartemisinin and
holotransferrin. Cancer Lett 1995;91:41–6.
[24] Sadava D, Phillips T, Lin C,
Kane SE. Tranferrin overcomes drug resistance to artemisinin in human
small-cell lung carcinoma cells. Cancer Lett 2002;179:151–6.
[25] Reizenstein P. Iron, free
radical and cancer. Med Oncol Tumor Pharmacother 1991;8:229–33.
[26] Shterman N, Kupfer B, Moroz C.
Comparison of transferrin receptors, iron content and isoferritin profile in
normal and malignant human breast cell lines. Pathobiology 1991;59:19–25.
Keywords: Artesunate; Dihydroartemisinin;
Tumor; Angiogenesis; Tube formation
*Corresponding author.
Tel.:
+86-571-87217206;
fax: +86-571-88075447.
E-mail address:
chenh552@163.com (H.-H. Chen).
Purchase
Artemisinin
Cancer Therapy
Products List |



