In Search of the Fountain of Youth
Preliminary Analysis of Deuterium’s
Role in DNA Degradation
Kirk B. Goodall
July 22, 2003
kgoodall2001@yahoo.com
Evidence indicates that aging is coupled to a gradual
accumulation of errors in DNA that arise due to strand breakage, DNA
replication errors, or dysfunctional DNA repair mechanisms. Therefore,
it is logical to consider factors that adversely impact DNA and explore
the effect of removing them from the cellular environment. Although
low-level solar radiation is the most pervasive mutagen known to adversely
affect DNA, deuterium oxide is also pervasive and known to adversely
affect DNA. Deuterium oxide is present in the Earth’s surface waters
at concentration of 155 parts per million (ppm). At this low level
it is generally thought to have no effect. However, over long periods
of time low levels of deuterium could play a role, especially when
coupled with radiation and other mutagens that lead to DNA damage.
New research indicates that although largely ignored, deuterium oxide
may play a key role in the aging process.
Aging Theories Tied to DNA
The theoretical maximum lifespan that an organism can achieve is tied to its DNA and its intra-cellular
processes. At the end of chromosomes is a strand of DNA known as a telomere. With each cell division
some of the telomere sequence is lost, which in turn limits the number of times a cell can divide. Leonard
Hayflick discovered that embryonic fibroblasts (connective tissue cells) could divide a maximum of about
50 times before the telomere is gone. (1) If cells continue to divide after loosing their telemeres, functional
DNA is lost and cells soon malfunction. Once the “Hayflick Limit” has been reached chromosome ends
begin sticking together and result in cells invoking apoptosis or senescent mechanisms, preventing
mutations. This effectively establishes an upper limit on lifespan.
Lifespan and the number of fibroblast doublings are connected as well. Fibroblasts in mice with a 3-year
maximum lifespan undergo 15 doublings, chickens with a 12-year lifespan undergo 25 doublings and the
Galapagos tortoise with a 175-year lifespan exhibits 130 doublings. (2) These species differ in initial
telomere length as well as in the number of telomeres lost in each cell division.

Fig. 1 Harriet, the world's oldest known living
resident. Age: 173 years, born around Nov. 15, 1830. Brought back from
the Galapagos Islands by Charles Darwin, as confirmed by DNA testing.
(3)
If other factors did not come into play before these limits are
reached, humans would perhaps live to be far
older. One of the prime limiting factors is naturally occurring damage
to DNA. In addition to being damaged directly by radiation, DNA is
damaged by free radicals produced by radiation, mutagens, and normal
metabolic processes. Accumulation of DNA Errors
There is substantial evidence that aging is tied
to a decline in the integrity of DNA. In research performed by Dr. Howard
J. Curtis of Brookhaven National Labs, mice were irradiated with sub-lethal
doses of radiation to test its long-term effects. This work was done
back in the 1960s as part of the Gemini Astronaut Program. Astronauts
are subjected to higher levels of radiation due to background space radiation
and the lack of shielding in lightweight space capsules. Although astronauts
were subjected to elevated radiation levels on Apollo missions, exposure
times were relatively short. On a two year manned mission to Mars, radiation
exposure becomes a significant problem.
 
Fig. 2 Effect of Radiation on Aging. “These
mice pictured above are all 14 months old. As young adults, nine mice
were given sub-lethal doses of radiation and nine others were left
as untreated controls. The control mice (left) are still sleek and
vigorous at 14 months, while six of the irradiated mice have died and
the remaining three show signs of extreme aging (right). [Research
photographs of Dr. Howard J. Curtis.]” (4)
The Earth's atmosphere provides shielding from a large percentage
of space radiation, but cosmic rays and energetic
protons from the sun still penetrate the atmosphere down to the Earth's
surface. This radiation in conjunction with natural terrestrial radioactive
sources produce ionizing radiation that adversely affects DNA. In humans
almost all DNA damage is repaired by effective repair mechanisms. However,
in some cases DNA repair mechanisms are dysfunction
and result in diseases that mimic the effects of aging.
Examples of
Dysfunctional DNA Repair.
Other clues that aging is DNA based may be found in rare inherited
diseases that occur when genes that maintain the integrity of the genome
are mutated. For instance when genes responsible for DNA repair are
corrupted the result may be premature aging causing effects such as:
wrinkled skin, gray hair, and shorten lifespan. In Werner's syndrome
hair turns gray after age 20 and by the late 40s the patient shows
signs of advanced aging such as cataracts, osteoporosis, and atherosclerosis.
Werner's syndrome is caused by mutations in WRN, which encodes a helicase
essential for maintenance of telomeres and DNA repair. Cockayne syndrome
is caused by mutations in genes involved in transcription-coupled DNA
repair. Although patients do not show signs of advanced aging, they
do suffer greatly reduced lifespans.
Error-Free DNA Repair
It is possible that aging may not be caused by DNA
mutations in general, but by mutations in genes
required for error-free repair and replication
of all DNA. In 1974 R. W. Hart and R. B. Setlow,
published their paper: “Correlation
between Deoxyribonucleic Acid Excision-Repair
and Life-Span in a Number of Mammalian Species” (5) in which they measured
the ability of fibroblasts to perform unscheduled
DNA synthesis after UV irradiation. Fibroblasts'
ability to perform unscheduled DNA synthesis is
a measure of excision-repair.
| Correlation between lifespan
and the relative effectiveness of DNA repair
in cells of certain mammals. In each case,
cells growing in tissue culture were irradiated
with ultraviolet light and then the efficiency
with which they repaired their DNA was determined.
(From the work of R. W. Hart and R. B. Setlow,
1974.) |
| Species |
Average lifespan, yr |
Effectiveness of DNA repair as measured
by the amount of unscheduled synthesis (grains/nucleus) |
| Human |
70 |
50 |
| Elephant |
60 |
47 |
| Cow |
30 |
43 |
| Hamster |
4 |
26 |
| Rat |
3 |
13 |
| Mouse |
2 |
9 |
| Shrew |
1 |
8 |
fig 3 “Aging
represents the inevitable consequence of a failure
of DNA repair” (4)
Hart and Setlow found that “both the rate and extent of unscheduled
DNA synthesis after UV irradiation of fibroblasts increases with the
life-span of the of the species.”(5) This model assumes that UV radiation
effectively mimics normal wear and tear in cellular DNA. Hart and Setlow
cautioned that there is “more to aging than just the failure of an
excision-repair system for dimmers.” (5) Nevertheless, these tests
show that error free DNA repair is essential for species with long
life spans.
Hitting the Aging Wall.
It is general accepted that aging is not a linear
process. The rate of aging increases with time with the result that
humans age more rapidly at the end of a typical lifespan. The body
can be viewed as an extremely complex feed back control system, employing
multiple feed back control loops. When one feed back loop is compromised
it can adversely affect others with the result that the entire system
begins to perform in a non-optimal fashion. When this effect is coupled
with a decline in the overall health of cells, the result can be a
critical failure, i.e. death. In 1963, Orgel published the seminal
paper on aging: The Maintenance of the Accuracy of Protein Synthesis
and its Relevance to Aging. (6) Orgel proposed that protein synthesis
will have some initial error rate Po and that the rate of error increase
will be proportional to some constant alpha.
The solution to this differential equation is:
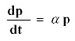
eq [1]
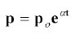
eq [2] This equation states that the rate of error accumulation in protein
synthesis grows exponentially over time. Orgel conservatively proposed
this model as one explanation for the progressive deterioration of
cells and not as a model for aging of the entire organism. However,
other aspects of the aging process also exhibit exponential growth,
such as cancer rate as a function of age.
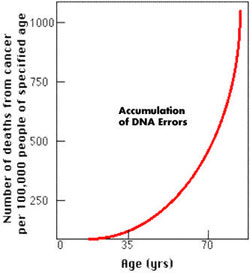
Fig. 4 Possible Link Between DNA Errors and Cancer Rates. “Cells
taken from old people (and people with premature aging syndromes)
show marked reductions in the transcription of many genes, especially
genes involved in DNA replication, DNA repair, and in checkpoints
that ensure accurate mitosis of the cell. Many of these changes
also cause cancer so it is no accident that the incidence of cancer
rises with advancing age.” (4)
Cancer and a general decline in the integrity of DNA are undoubtedly
related. An accumulation of DNA errors likely increases
exponentially over time. This indicates that towards the end of
life, one “hits
a wall” where the integrity of DNA has been so compromised, that no
method short of reordering all DNA would significantly
extend life. At this juncture the quality of life is likely to be
so severely diminished that there is no point in extending life further.
Therefore a premium exists on maintaining the integrity of DNA starting
early in life, so that the overall length and quality of life is optimized.
Ionizing Radiation Threshold for DNA Degradation
Literature addressing the biological effects of low-level radiation
shows that gradual changes to DNA induced by radiation and other
mutagens are below the threshold of current detection methods. It
is established that acute radiation exposure will lead to premature
aging, but the debate continues as to whether or not normal levels
of radiation exposure lead to mutations. Ionizing radiation produces
free radicals that attack DNA, but so do normal metabolic processes.
Some groups even claim that low-level radiation triggers an “immune
response”, which protects DNA from further damage. Options on the
subject seem to be influenced by political and financial considerations.
Normal levels of ionizing radiation have little adverse effect,
but it only takes a small number of mis-repaired strand breaks for
DNA damage to accumulate over decades of exposure, and result in
aging effects.
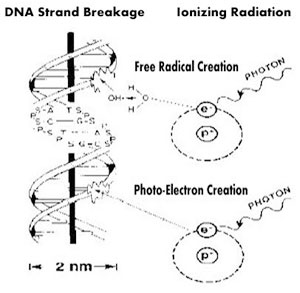
Fig. 5 Two Mechanisms of Strand Breakage Linked to Ionizing Radiation
What is unique about ionizing radiation relative to other mutagens
is that it creates clusters of ionizations and
reactive chemical agents on the scale of the DNA molecule. Dudley
Goodhead has found that “this clustering occurs, even at the lowest exposures, within
a pattern of ionized and excited molecules along the path (or ‘track')
of an individual particle.” (7) Goodhead's research has revealed
that “a high proportion of the DNA damage is complex, even from
sparsely-ionized radiations, including combinations of several strand
breaks and base damages (ie considerably more complex than clean
double-strand breaks); these severe damages present a special challenge
to the cell's repair systems, and it has been hypothesized that
they may dominate the long-term consequences of the irradiation.” (7)
What is beyond question is that double stand breaks
produced by ionizing radiation are more difficult to repair than
single strand breaks produced by free radicals. It is likely that
ionizing radiation impinging upon DNA during the replication phase
results in damage similar to a double strand break and is also difficult
to repair. Therefore, any factor that slows down the rate of DNA
replication could expose DNA strands to a greater chance of corruption
from ionizing radiation. Deuterium, Mitosis, & DNA.
It is known that high-levels
of deuterium slow down the rate of mitosis, but the exact mechanism
remains unknown. In 1989 Jan Lamprecht, Dieter Schroeter, and Niedhard
Paweletz conducted a study on deuterium's effect on mitosis at The
Institute of Cell and Tumor Biology and German Cancer Research Center
in Heidelburg. (8,9) In one test, cells were subject to to 25%,
50% and 75% deuterium oxide for two hours. Another test was performed
in which cell were subjected cells to 75% heavy water for two, six,
twelve, and twenty-four hours. The percentages of cells in prophase,
metaphase, anaphase, telophase, and interphase were then measured.
The data showed abnormally high numbers of cells in prophase and
metaphase and especially in metaphase. If DNA replication rate is
slowed down during the prophase, then ionizing radiation could break
strands when they are most susceptible to corruption. However, to
verify this effect new tests must be performed with no deuterium
present in DNA molecules and enzymes to determine if replication
rates are increased over DNA containing normal deuterium concentrations.
Hydrogen and Deuterium Bonds in DNA
Deuterium is thought to have an effect on biological processes
through the mechanism of hydrogen bonding. Hydrogen bonds play a
role in DNA structure and are partially responsible for the double
DNA strand assuming a helical shape. The hydrogen bonds created
by a deuterium atom are stronger than a normal hydrogen atom.
Hydrogen Bond Strength.
The presence of a neutron in the nucleus
of the hydrogen atom doubles the atomic mass and thereby decreases
the intermolecular vibration frequency. (10) This has the effect of
increasing the hydrogen bonding strength. The physical properties
of deuterium oxide differ only slightly from those of hydrogen oxide,
or normal water. In aqueous solutions the hydrogen bond strength of
deuterium oxide is greater by approximately 0.24 kcal/mol, which represents
about a 6% increase over pure water. (10). The hydrogen bond strength
in organic compounds is typically lower and is difficult to measure
directly. However, by employing vibration mode partition functions
from statistical thermodynamics, an accurate model may be constructed
to calculate physical and chemical properties of deuterium oxide.
Martin Cuma and Steve Scheiner made use of Gaussian codes to calculate
in the increase in bonding strength due to substitution of deuterium
for hydrogen within common organic groups. (11)
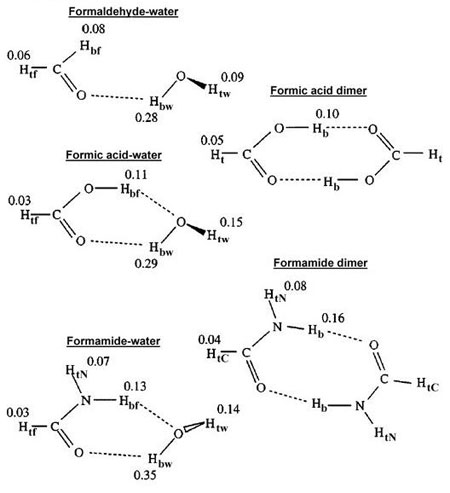
Fig. 6 The effect of deuterium substitution on hydrogen bond strength
as appearing in: Influence of Isotopic Substitution on Strength
of Hydrogen Bonds of Common Organic Groups (11) Dashed lines are
hydrogen bonds. Substitution of deuterium for hydrogen has a comparatively
greater effect on the overall bonding strength when the atom directly
participates in a hydrogen bond. Values shown are in kcal/mol.
Organic Compound |
H-Bonds |
Min H-Bond D-Sub |
Crit H-Bond D-Sub |
Max H-Bond D-Sub |
Formaldehyde-water |
1.60 kcal/mol |
0.06 kcal/mol |
0.28 kcal/mol |
0.50 kcal/mol |
Formic acid dimer |
10.84 kcal/mol |
0.05 kcal/mol |
0.10 kcal/mol |
0.29 kcal/mol |
Formic acid-water |
5.97 kcal/mol |
0.03 kcal/mol |
0.29 kcal/mol |
0.57 kcal/mol |
Formamide dimer |
8.32 kcal/mol |
0.04 kcal/mol |
0.16 kcal/mol |
0.55 kcal/mol |
Formamide-water |
4.52 kcal/mol |
0.03 kcal/mol |
0.35 kcal/mol |
0.68 kcal/mol |
Table 1 The combined
strength of the H-Bonds is modified by the presence
of deuterium atoms in H-Bonding sites. Substitution
of some hydrogen atoms with deuterium atoms have
very little effect such as those shown under the
column Minimum H-Bond D-Sub. Others have a large
effect, such the values shown under the column
Critical H-Bond D-Sub. The maximum effect occurs
when all hydrogen atoms are replaced by deuterium
atoms as indicated under the Max H-Bond D-Sub
column.
Organic
Compound |
Min
H-Bond D-Sub Incr |
Crit H-Bond
D-Sub Incr |
Max
H-Bond D-Sub Incr |
Formaldehyde-water |
3.75% |
17.50% |
31.25% |
Formic acid
dimer |
0.46% |
0.92% |
2.76% |
Formic acid-water |
0.50% |
4.85% |
9.54% |
Formamide dimer |
0.48% |
1.92% |
6.61% |
Formamide-water |
0.66% |
7.75% |
15.04% |
Table
2 The percentage increase of bonding strength
over the normal H-Bond strength is shown the
table above based upon the values reported in
Table 1.
Hydrogen Bonds in DNA.
In DNA the hydrogen bonds of interest are the G-C
and A-T bonds that form between the strands of the
double helix. The exact value for the strength of
deuterium bonds in DNA is difficult to assess. Estimates
of the strength of individual hydrogen bonds in
DNA have been made by Turner and Sugimoto, but there
is debate over the accuracy of their model. (12)
Griffiths reports that deuterium bonds in enzymes
that act upon DNA are typically 0.4 to 1.7 kJ/mol
stronger than for normal hydrogen bonds. (13) The
substitution of deuterium for hydrogen with DNA
undoubtedly affects the bond strength, however,
determining the degree of this effect is very difficult
and can only be approximated by computationally
intensive numerical methods.
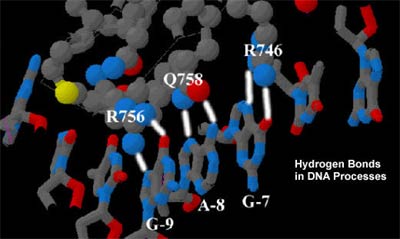
Fig. 7 Example of Hydrogen Bonds in DNA Processes. “RNA
polymerase transcribes specific genes that are
found in the DNA. The polymerase recognizes
these genes because they have PROMOTERS. A
PROMOTER is an RNA polymerase binding site in the
DNA which comes just before a gene. For T7 RNA polymerase,
the DNA sequence that makes up the promoter is TATAGTGAGTCGTATTA
in the template strand. RNA Polymerase recognizes
the promoter sequence by hydrogen bonds: Arginine
756 makes two hydrogen bonds to Guanine-9, Glutamine
758 makes two hydrogen bonds to Adenine-8, and
Arginine 746 makes two hydrogen bonds to Guanine-7.
These are only a few of the protein-DNA interactions
involved in promoter recognition”. (14)
Length of Hydrogen Bonds.
Of prime importance
in DNA replication and repair is the shape of
enzyme molecules that govern these processes.
Deuterium shortens the bond length slightly and
may inhibit proper functioning of enzymes. However
this effect is likely to be small, on the order
of perhaps a 1% change. In general, hydrogen bonding in DNA is a cooperative
process that effects stacking interactions and involves
the entire molecule. If we use enzymes as a guide,
the bond strength of deuterium in DNA is perhaps
0.5% to 2% greater than for hydrogen. When considering
the small concentration of deuterium found in nature,
and the slight increase in the strength of deuterium
bonds over hydrogen bonds, one might be tempted
to conclude that deuterium has no appreciable adverse
effect on DNA at a concentration of 155 ppm. However,
in addition to increased hydrogen bonding strength,
deuterium has other potentially adverse effects.
Plausible Mechanisms for Deuterium Adversely
Effecting DNA
Deuterated Enzymes: As early as 1974,
deuterium was advanced as a possible cause of
aging. One notable theory is that deuterium adversely
affects the shape of enzyme molecules, which are
involved in DNA processes.
This is the central
concept advanced by Griffiths in: The Possible
Roles of Deuterium in the Initiation and Propagation
of Aging and Other Biological Mechanisms and
Processes;
“When deuterium is involved in a chemical
reaction, consideration must be given to a slight
change in the inductive effect, as deuterium
is more electronegative than hydrogen. Hyperconjugative
effects are also involved since CD3, for example,
is less delocalized than
CH3, and, more important, the effective size
of a C-D bond is smaller than the effective
size of a C-H bond. Thus steric effects have
a part to play, reinforcing our contention
that any highly stereospecific enzyme molecule
containing a deuteron in an important
position has a potential for participating in
an error reaction.” (13)
Deuterium Compromised DNA Repair Enzymes
A
large class of enzymes and proteins play a role
DNA replication and repair. (15) Some enzymes
used during DNA replication and repair make extensive
use of hydrogen bonds. These enzymes are potentially
highly susceptible to adverse effects from deuterium
contamination. One notable protein is p53, which
plays a significant role in DNA repair. “Several
different types of DNA damage can activate p53,
including double-strand breaks in DNA produced
by gamma-irradiation and the presence of DNA repair
intermediates after ultra-violate irradiation
or chemical damage to DNA.”(16) It is interesting
to note that over 50% of human cancers contain
mutations in the gene that produces the p53 protein.
Slowing of DNA Replication
Deuterium
could also inhibit an enzyme such as DnaB, which
is responsible for unwinding and separating DNA
during replication. Other enzymes such as Primase
and polymase play key roles in synthesizing RNA
and in adding nucleotides to the DNA chain during
replication. If any of these enzymes are inhibited,
the rate of DNA replication could be slowed appreciably
when the DNA is most susceptible to damage by
radiation. Thus deuterium could also act as a
catalyst for DNA degradation when coupled with
common levels of radiation exposure.
Bonding Site Inhibition of DNA Repair
The
process of DNA replication is in some ways similar
to the repair of a double strand break. Complex
enzymes detect breaks and rejoin DNA strands based
upon specific bonding sites. Hydrogen bonds are
often employed within these binding sites. If
deuterium is present in these sites, steric effects
and increased bonding strength could also inhibit
DNA repair. Once again, if the rate of DNA repair
is severely curtailed, ionizing radiation could
further disrupt the repair process while the strand
is broken and is susceptible additional radiation
damage.
Deuterium Studies on Organisms
Fully deuterated water, known as heavy water,
is toxic. It is primarily used as a moderating
agent in nuclear power plants. Largely due to
the fact that heavy water is readily available,
but deuterium depleted water (DDW) is scarce,
relatively few studies have been conducted on
the biological effects of DDW verses heavy water.
It would normally stand to reason that if increasing
deuterium levels above those found in nature has
no measurable effect, then decreasing deuterium
levels will have no effect either. However, one
must considered that deuterium has been in the
environment for a long time , and therefore
it is possible that through evolution, humans
and other organisms have developed mechanisms
to protect against it, or eliminate it. In a manner
somewhat analogous to a pH buffer, cells may be
able to moderate the adverse effects of increased
levels of deuterium. That the human body has some
means of eliminating deuterium is evidenced by
the fact that deuterium levels in the body are
typically 80% of that found in the nature. It
stands to reason that if deuterium had no harmful
effect on the body, the body would not attempt
to expel it. Finally, one must considered that
all organisms on the planet are exposed to low
levels of deuterium throughout their entire lifetime.
In the absence of a control group, the effects
may not be obvious.
Deuterium Studies with Algae
Much of
the work studying deuterium's effect on biological
organisms was performed at Argonne National Laboratory
in the 1960s. Work continues to this day primarily
on various strains of blue-green algae. Most algae
may be grown in 100% heavy water, but at a significantly
reduced rate as compared to within tap water.(17)
An organism's success in adapting to growth in
pure heavy water is to some degree tied to the
complexity of its DNA. Organisms with relatively
small genome sizes, (on the order of 3-4 million
base pairs for algae and bacteria) and that lack
sophisticated DNA repair mechanisms, (18) can
successfully adapt to growth in pure deuterium
oxide. Mammals, such as mice, with genome size
on the order of 3 billion base pairs cannot have
more than 25% of their body water replaced with
deuterium before going into convulsions. (19)
Mice Studies with High Levels of Deuterium
Studies
have been performed on mice in which they consumed
deuterium at a 20% to 30% concentration, after
which they were irradiated with at near lethal
levels. (20) In one series of tests mice were
deuterated for 12 days after which they were exposed
to 8.5 Grays of radiation. In one case mortality
was significantly less in deuterated than in non-deuterated
mice. In another test “mortality from whole-body
neutron-boron radiation, unlike mortality from
whole-body x-radiation, was not lowered by deuteration.” (21)
These test were done to evaluate the use of deuterium
and radiotherapy in the treatment of malignant
human tumors. It is possible that by inhibiting
cell division high levels of deuterium could protect
against short-term radiation exposure. However,
there are adverse side effects from such elevated
levels of deuterium, which limit its clinical
application.
Hungarian and Romanian Deuterium Studies
Only in the last ten years has a program been
developed to study the effects of less deuterium
on the human body. Most of these studies have
been performed in Hungary and Romania where low
cost deuterium depleted water is available. The
studies have involved cancer in both humans and
animals.
Radiation and Low Level Deuterium Studies
on Mice
Of particular note is a study performed
by W. Bild, et al at the Romanian University
of Medicine and Pharmacy. (22) In this study
mice were fed DDW at a 30 ppm concentration
for a 15 days period during which they were
exposed to a sub-lethal dose of 8.5 Grays of
radiation. A control group of mice were fed
tap water and exposed to same level of radiation.
The test group had a survival rate of 61% while
the control group's survival rate was 25%. The
test group also maintained normal white blood
cell and red blood cell platelet counts as compared
to the control group. Test group mice that were
infected with K. pneumonia 506 and S. pneumonia
558 in addition to being irradiated or treated
with cyclophosphamide showed increased non-specific
immunity parameters. Test results generally
showed an intensification of the immune defenses
and increased proliferation of the peripheral
blood cells over the control group, which may
accounted for some of the radiation protective
effects. This test was done to evaluate the
effects of DDW on chemotherapy patients. However,
the results may also point to a relationship
between the adverse effects of radiation and
deuterium as they relate to aging.
Possible Mechanisms for Observed Effects on
Mice
As outlined earlier, deuterium slows
down the rate of mitosis and can conceivably
have adverse effects during the S phase of cell
division when DNA is most susceptible to radiation.
A radiation dose of 8.5 Grays would have resulted
in double strand breaks as well as greatly disrupted
DNA processes during replication. It is conceivable
that mice with lower levels of deuterium in
their systems would have been benefited from
less error prone cell division and more effective
repair of radiation damaged DNA. Such effects
could be viewed as a greatly accelerated case
of what happens over a lifetime of exposure
to solar radiation. Even at very low levels,
deuterium could slow down DNA replication processes,
or otherwise interfere with the repair of DNA
damaged by solar radiation. Over a lifetime
of exposure to low levels of both deuterium
and solar radiation , errors could accumulate
in DNA and contribute to aging.
Deuterium Depleted Water Trials on Cancer
Patients in Hungary
Although there is relatively little data on the
effects of DDW on healthy people*, there is a
wealth of data on the effects of DDW on cancer
patients. Gabor Somlyai has been successfully
using DDW to treat cancer in Hungary for the past
ten years. (23) Results have come from double-blind
clinical trials, and compassionate use of DDW
as an adjuvant treatment.
Clinical data appearing in: “The Biological
Effects of Deuterium Depletion” by Gabor Somlyai.
“Interim
evaluation confirmed a significant difference
between the control group and treated groups
with respect to the examined parameters that
indicated the anti-tumor effect of the preparation."
a) At the time of the 5 th and
6 th visits, the ratio of patients
showing an increased efficacy (PR) was significantly
higher statistically (5 th visit: p=0.0096;
6 th visit p = 0.021 in the treated group.
b) The
volume of the prostate decreased significantly
(p =0.043) in the treated group, whereas it
could be regarded as unchanged in the control
group.
c) The number of patients with a decreased
prostate volume was significantly higher (exact
Armitage-test: p=0.015; exact Fisher-test: p
=0.011).
d) Significantly more patients reported
a positive chance in symptoms in the treated
group (exact Armitage-test: p=0.0009; exact Fisher-test:
p=0.0018).
e) The survival rate in the treated group was
significantly higher (p=0.030)
After the consumption
of more than 10 tons of Dd-water no event endangering
life occurred. We did not experience any deterioration
in blood counts, irritation of the mucous membrane,
nausea, headache, etc., that could have been
attributed to Dd-water consumption. Compassionate use and as an Adjuvant Treatment:
Prior to and parallel to the above trials,
between October 1992 and the spring of 1999,
we provided Dd-water to approximately 1200 patents.
Our knowledge concerning the efficacy and application
methods of Dd-water comes mainly from the follow-up
of this patient population … During the last
8 years we provided about 350 tons of Dd-water
to the patients and some 12-14 thousand pages
of documentation records the data of the meticulous
follow-ups.
Recommendations, comments, dosage
advice, and results are based on these observations. Between October of 1992 and December of 1997,
887 patients began to consume Dd-water. Among
them, 134 patients (15 percent) were diagnosed
with breast cancer. The ratio show that patients
with breast cancer were represented in an approximately
equal ratio among patients consuming Ddwater,
to that of the entire population (in the US
, for example, 13 percent of all cancers is
that of the breast)” (23)
* Note: For the past three months the author
has been consuming 36 liters of DDW per month
at a 105 ppm concentration with no ill effects.
The author has experience a slight increase in
stamina during strenuous exercise, but this effect
is hard to quantify or verify.
Trials with DDW-25
will begin soon.
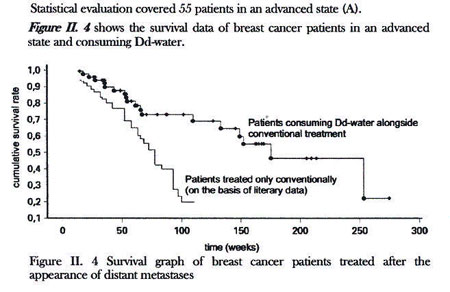
Figure II. 4 that 85 percent of the above
population survived one year after the beginning
of the Dd-water consumption in an advanced state,
56 percent survived 3 years, 47 percent 4 years,
and 25 percent 5 years after the beginning of
consumption of Dd-water.” (23)
Thus there is plenty of clinical evidence that DDW
consumption does have a measurable effect on cancer
and since cancer and aging are related processes,
there is a good chance that DDW consumption could
have an anti-aging effect.
Discarded Hunza Data Revisited
Although deuterium levels in glacier fed streams
is known to be less than what is found in surface
water, the effect is diminished by rainwater entering
streams as they flow down to cultivated areas.
There have been numerous claims of people in mountainous
regions living exceedingly long lifetimes, even
approaching 150 years, but few of these reports
have been validated. There was interest in the
Vilcabamba region of Ecuador , the Caucasus region
of Russia , and the Hunza region of Pakistan due
to an article by Dr. Alexander Leaf in the January
1973 issue of National Geographic. (24) However,
the Vilcabamba cases were refuted by R.B. Mazess
in 1979 and in 1982. (25,26) Likewise, the longevity
claims of the Caucasus region arose due to age
exaggeration on the part of individuals attempting
to avoid military service. The politics of the
former Soviet Union also fostered an image of
superiority of people from the region where Stalin
was born. (27) Indeed, the geography of these
regions does not promote low deuterium levels
in melt water runoff and does not fit the model
proposed here. However, the Hunza region is different
in this regard. Unlike the Vilcabamba and Caucasus
regions, the Hunza region receives very little
annual rainfall, only about 4 inches. (28) The
Hunza people receive their drinking water from
glacier runoff, which is also used to irrigate
their crops. The area is in may ways a high altitude
desert. Although published deuterium levels for
the Hunza region could not be found, we can make
a very good estimate based upon “Deuterium Content
of Stream Waters of Glacier Origin in the Himalayas ” by
Hisao Wushiki. (29)
Himalayan Geological Studies Involving Deuterium
In
his 1977 Glaciological Expedition to Nepal , Hisao
Wushiki measured the deuterium content in a number
of streams that feed the Sun Kosi River . The
levels vary by time of the year and location.
Streams at higher elevations during the winter
and post-monsoon seasons have the lowest deuterium
levels. The values ranged from –66 SMOW (Standard
Mean Ocean Water, this scale ranges from 0 at
158 ppm to –1000 at 0 ppm.) for the Sun Kosi river
to -170 SMOW for the Ronabuk tributary located
on the backside of Mt. Everest, farthest away
from India. Much of the snow in the Himalayas
arises from moist air that flows up from India
during monsoon season. This water is rich in deuterium,
but as it passes over the mountains the heavy
water precipitates out first, leaving deuterium
depleted water, which falls as snow in the higher
elevations. 
Fig. 8 Terraced Hunza Fields Irrigated by Glacier
Water Containing Lower Levels of Deuterium
Inferred Hunza Deuterium Level
Based
upon geography and altitude we can infer that
the deuterium content of the Hunza region is
comparable to the runoff from the glaciers associated
with Mt. Gosainthan which is at an altitude of
8013 meters and located approximately 700 km inland.
Runoff from the Gosainthan glaciers is at –160
SMOW, which corresponds to 133 ppm or about
a 16% reduction over normal surface water. The
Hunza people receive their water from the glaciers
of Mt. Ultar , which is at an attitude of 7398
meters and located 1600 km inland. For this
reason Hunza water most likely has a deuterium
concentration at or below 133 ppm. A 16% reduction
may not seem significant, however, the Griffiths
theory predicts that the adverse biological
effect of deuterium goes by the square of the
concentration. (13) Although the true age of
the Hunza people is difficult to verify, there
is ample evidence to support the claim that
the elderly people of the region are vigorous
and long lived. This has been loosely tied to
diet and exercise, but it could also be attributed
to lower deuterium levels in the water and food
of the region.
Effectiveness of Consuming DDW
Consumption of DDW differs from antioxidant formulas,
HGH stimulants, vitamins, and other anti-aging
remedies in one key aspect: DDW does not change
its chemical composition when digested. All DDW
consumed has a direct effect at the cellular level.
However, for DDW to have a measurable impact,
existing deuterium must first be leached out of
the body. Through thermal substitution reactions
deuterium atoms are replaced over time by regular
hydrogen atoms. This process proceeds fastest
when the concentration of deuterium in drinking
water is at the lowest possible level. Unless
food that is consumed is also grown with DDW,
there will always be deuterium in the diet. Consumption
of DDW can conceivably protect DNA from damage
and assist DNA repair mechanisms, but it does
not directly repair DNA. It is therefore questionable
whether DDW consumption will “rejuvenate” the
body, but it certainly could serve to protect
the body and enable it to function more efficiently.
Work in Progress & Suggested Research
At this writing a sample of Hunza water is being
collected from streams close to the glacier source.
Within 60 days this sample should be available
in the United States for deuterium level testing
by an independent lab. This will help to establish
actual deuterium levels in glacier runoff within
the Hunza region.
When the theory of enzymes compromised by deuterium
was advanced in 1974, many of the tools to explore
the DNA at the molecular level were not available.
A highly desirable experiment would be to deuterise
key enzymes involved in DNA replication and repair
and then determine if the rate of these processes
is significantly inhibited.
As of July 2000 researchers at Brookhaven National
Labs published reports highlighting new technology
for assessing damage done to DNA by double strand
breaks. Special enzymes are used to cut DNA at
sites exhibiting specific kinds of damage. These
segments are then separated and counted on electrophoretic
gels to measure clusters of damaged DNA. Such
tests could be repeated using partially deuterated
DNA, or fully deuterated DNA taken from algae
grown in heavy water.
For more information on Deuterium water visit www.hydros.com
References
• 1. Shay, JW and WE Wright, Hayflick,
his limit, and cellular aging . Nature Reviews.
Oct. 2000, Vol.1:73-76
• 2. Goldstein, S. Growth of cultured
cells from the Galapagos tortoise , Exp.
Cell. Res. 83:279-302
• 3. Prof. Jan M.L. Martin, Department
of Organic Chemistry Weizmann Institute of Science,
IL-76100 Rehovot , Israel http://www.weizmann.ac.il/~comartin/harriet.html
• 4. John W. Kimball , “Biology”,
Addison Wesley, January 1983 Kimball's Biology
Pages: http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/A/Aging.html
• 5. R.W. Hart, R.B. Setlow, Correlation
Between Deoxyribonucleic Acid Excision-Repair
and Life-Span in a Number of Mammalian Species ,
Proc. Nat. Acad Sci, USA, Vol. 71, No. 6, 2169-2173,
June 1974
• 6. L.E. Orgel, The Maintenance
of the Accuracy of Protein Synthesis and Its
Relevance to Aging , Biochemistry, Vol.
49, 517-521, 1963, February 15, 1963
• 7. Goodhead, D.T. MRC Harwell, Radiation
and Genome Stability Unit http://www.ragsu.har.mrc.ac.uk/damage/index.htm
• 8. Lamprect, J., European Journal
of Cell Biology 51:(2) 303-312 " Mitosis
Arrested By Deuterium Oxide - light microscopic,
immunofluorescence and ultrastructural characterization "
Stuttgart , Germany : Wissenschaftliche Verlag GMBH, April 1990.
• 9. Schroeter D., European Journal
of Cell Biology 58:(2) 365-370 " Deuterium
Oxide Arrests the Cell-Cycle of PTK2 Cells During
Interphase " Stuttgart , Germany : Wissenschaftliche
Verlag MBH, August 1992.
• 10. George Nemethy, Harold A. Scheraga, “ Structure
of Water and Hydrophobic Bonding in Proteins.
IV. Thermodynamic Properties of Liquid Deuterium
Oxide ”, Journal of Chemical Physics, Aug.
1964, Vol 41, No. 3, 680-689
• 11. Martin Cuma, Steve Scheiner, “Influence
of Isotopic Substitution on Strength of Hydrogen
Bonds of Common Organic Groups”, Journal
of Physical Organic Chemistry, Vol. 10, 383-395,
1997
• 12. Turner, Sugimoto, Free Energy
increments for hydrogen-bonds in nucleic acid
base pair , J. Am. Chem. Soc. 109,3783-85
• 13. T. Redston Griffiths, The Possible
Roles of Deuterium in the Initiation and Propagation
of Aging and Other Biological Mechanisms
and Processes ,
Proceedings of the Second International Conference
on Stable Isotopes, October 20-23, 1975 Oak Brook
Illinois
• 14. Dr. John Barnard, Department
of Microbiology State University of New York at
Buffalo http://www.acsu.buffalo.edu/~jbarnard/rnap.html
• 15. R.D. Wood, M. Mitchell, J. Sgouros,
T. Lindahl, Human DNA Repair Genes , Science,
Vol. 291, 1284-1289, February 16, 2001
• 16. Levine, Arnold J, p53 the
Cellular Gatekeeper for Growth and Division ,
Cell, Vol. 88, 323-331, February 7, 1997
• 17. Joseph J. Katz, Chemical
and Biological Studies with Deuterium, Thirty-Ninth
Annual Priestley Lectures, Pennsylvania State
University, April 26-29, 1965
• 18. Malhotra K., Kim S.-T., Batschauer
A., Dawut L., Sancar A., Putative blue-light
photoreceptors from Arabidopsis thaliana and Sinapis
alba with high degree of sequence homology to
DNA photolyase contain the two photolyase cofactors
but lack DNA repair activity . Biochemistry
34: (1995) 6892-6899.
• 19. Thomson J.F. Biological Effects
of Deuterium , New York , New York , The
Macmillan Company, 1963
• 20. Laissue JA, Bally E, Joel DD,
Slatkin DN, Stoner RD, Protection of mice from
whole-body gamma radiation by deuteration of drinking
water , Radiat Res 1983 Oct;96(1):59-64
• 21. Slatkin DN, Stoner RD, Gremme
AM, Fairchild RG, Laissue JA, Whole-body irradiation
of deuterated mice by the 10B(n, alpha)7Li reaction ,
Proc Natl Acad Sci USA 1983 Jun; 80(11):3480-4.
• 22. Bild W, Stefanescu I, Haulica
I, Lupusoru C, Titescu G, Iliescu R, Nastasa V., Research
Concerning the Radioprotective and Immunostimulating
Effects of Deuterium-depleted Water , Romanian
Journal of Physiology, 1999 Jul-Dec; 36(3-4):
205-18
• 23. Gabor Somlyai, The Biological
Effects of Deuterium Depletion , HYD Ltd.,
2001, ISBN:0-7596-9261-0
• 24. Alexander Leaf, M.D., Every
Day Is a Gift When You Are Over 100 , National
Geographic, January 1973
• 25. Mazess RB; Forman SH, Longevity
and age exaggeration in Vilcabamba, Ecuador .,
Journal Gerontol 1979 Jan;34(1):94-8
• 26. Mazess RB; Mathisen RW, Lack
of unusual longevity in Vilcabamba, Ecuador ,
Human Biology, 1982 Sep;54(3):517-24
• 27. Aeiveos Corporation http://www.
aeiveos.com/longevity/
• 28. http://www.mysiliguri.com/darjeeling/climate.htm
• 29. Hisao Wushiki, Deuterium
Content of Stream Waters of Glacier Origin in
the Himalayas , Glaciological Expedition
of Nepal , Contribution No. 37, 40-42, 1977,
National Snow and Ice Data Center , World Data
Center for Glaciology, Phone: 303-492-4004
Additional References
Perry A. Frey, Strong Hydrogen Bonding in
Molecules and Enzymatic Complexes, Magnetic
Resonance in Chemistry , 2001, Vol 39, 190-198
S. Scheiner, M. Cuma, Relative Stability of
Hydrogen and Deuterium Bonds , J. Am. Chem.
Soc., 199, 1511-1521.
Ioannis Vakonakis, Miguel Salazar, Mijeong Kang,
Kim R. Dunbar & Andy C. LiWang, Deuterium
Isotope Effects and Fractionation Factors of Hydrogen-Bonded
A:T Base Pairs of DNA , Journal of Biomolecular
NMR, 25: 105-112, 2003 |



